Introduction:
While pediatric-inspired regimens are preferred in younger adults with Ph-negative ALL, the optimal approach for treating adults age >40 with Ph-negative ALL has not yet been defined. In the U.S., hyperCVAD-based regimens are commonly used in this age group; however, hyperCVAD may be less effective than pediatric-inspired regimens in patients who are appropriate candidates for intensive therapy, and may be excessively toxic in patients who are poor candidates for intensive therapy. We explored giving two different multiagent chemotherapy regimens to adults age >40 with newly diagnosed Ph-negative ALL, depending on the individual patient's age and fitness level.
Methods:
At our institution starting in September 2020, patients between 40-60yo with good performance status received an intensive pediatric-inspired regimen based on the CALGB 10403 backbone, while patients >60yo (or 50-60 with poor PS) received a moderate-intensity multiagent chemotherapy backbone consisting of 2-part induction (induction-1: infusional doxorubicin/vincristine D1-4 + dexamethasone 40mg D1-4, 9-12, 17-20; induction-2: same as induction-1 plus cyclophosphamide 1000mg/m 2 on day 1) and consolidation with 6 cycles of chemotherapy, alternating between methotrexate (1g/m 2) plus peg-asparaginase (1,000U/m 2), and cytarabine (1g/m 2 x 6 doses).
Prior to September 2020, patients in this age group received the hyperCVAD backbone. In both cohorts, patients not proceeding to allogeneic SCT in CR1 received POMP maintenance for up to 2 years.
We reviewed charts of patients aged >40 with newly diagnosed Ph-negative ALL at University of Colorado Hospital from 2014-2023 to compare outcomes between patients treated before 09/2020 (“hyperCVAD” cohort) and after 09/2020 (“age-adapted” cohort).
Results:
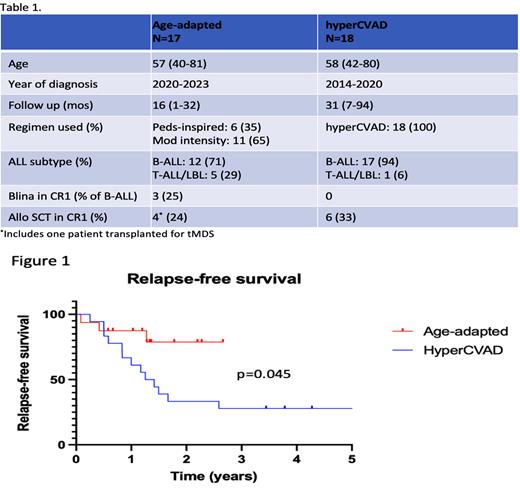
Between 09/2020-04/2023, 17 adults with median age of 57 (range 40-81) with newly diagnosed Ph-negative ALL began treatment at our institution. 6 patients (age 40-56) received a modified pediatric-inspired regimen, and 11 patients (age 54-81) received a moderate-intensity regimen. 4 patients underwent allogeneic SCT in CR1 (including 1 for tMDS that developed during maintenance therapy). 3 patients received blinatumomab in CR1, 2 of whom subsequently received allo SCT. With a median follow-up of 16 months (range: 1-32 months), 1 patient relapsed during maintenance and 2 patients died from treatment-related mortality (1 during induction chemotherapy, 1 during salvage therapy for primary refractory disease). All three events occurred in patients who received the moderate-intensity regimen. Grade 3 or 4 asparaginase-related toxicities occurred in 2 patients (1 gr3 hyperbilirubinemia, 1 gr3 pancreatitis) and both resolved with supportive care.
Between 2014-2020, 18 adults with median age of 58 (range 42-80) received hyperCVAD for newly diagnosed Ph- ALL. 6 patients received allogeneic SCT in CR1, and no patients received blinatumomab in CR1. With a median follow-up of 31 months (range: 7-94 months), 7 patients relapsed during consolidation or maintenance therapy and 3 relapsed after SCT. Among 10 patients who died during follow-up, 7 deaths were due to relapsed/refractory disease and 3 were due to treatment-related mortality (all 3 TRM were post-transplant).
An improvement in relapse-free survival is seen in the cohort treated with age-adapted chemotherapy vs. the hyperCVAD cohort (p=0.045, log-rank test) (Figure 1), while there was no difference in overall survival (p=0.29) (not shown).
Conclusions:
Age-adapted multiagent chemotherapy is safe and effective in adults age >40 with Ph-negative ALL and may result in better outcomes than hyperCVAD. This is possibly due to better efficacy of pediatric-inspired chemotherapy for younger (40-60yo) patients and better tolerability of moderate-intensity chemotherapy for older (>60yo) patients, compared to hyperCVAD. Broad use of asparaginase in both the pediatric-inspired and moderate-intensity regimens may have also contributed to better outcomes in comparison to hyperCVAD.
Incorporation of novel therapies in the frontline setting holds much promise to improve outcomes for older adults with Ph- ALL. Our observations suggest that the specific multi-agent chemotherapy regimen used, and in particular the incorporation of peg-asparaginase, may also affect outcomes significantly.
Disclosures
Schwartz:Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy. McMahon:Syros Pharmaceuticals: Research Funding; Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Arcellx: Membership on an entity's Board of Directors or advisory committees; Syndax Pharmaceuticals: Research Funding. Pollyea:Teva, Karyopharm, Bristol Myers Squibb, and AbbVie.: Research Funding; AbbVie, Bristol Myers Squibb, Syros, Novartis, Beigene, Bergen Bio, Arcellx, Jazz, Genentech, Immunogen, AstraZeneca, Kura, Ryvu, Magenta, Qihan, Zentalis, Medivir, Hibercell, LINK, Daiichi Sankyo, Aptevo, Rigel, Sumitomo, Adicet, Seres, Gilead, OncoVerit: Consultancy. Smith:OncoVerity: Current Employment, Current holder of stock options in a privately-held company; AML JV: Consultancy. Jordan:AML JV: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal